E. V. Katkova, A. V. Onufriev, B. Aguilar, and V. B. Sulimov. Accuracy comparison of several common implicit solvent models and their implementations in the context of protein-ligand binding. Journal of Molecular Graphics and Modelling, 72:70–80, 2017. [ DOI ]
Abstract
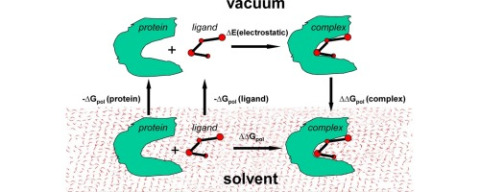
In this study several commonly used implicit solvent models are compared with respect to their accuracy of estimating solvation energies of small molecules and proteins, as well as desolvation penalty in protein-ligand binding. The test set consists of 19 small proteins, 104 small molecules, and 15 protein-ligand complexes. We compared predicted hydration energies of small molecules with their experimental values; the results of the solvation and desolvation energy calculations for small molecules, proteins and protein-ligand complexes in water were also compared with Thermodynamic Integration calculations based on TIP3P water model and Amber12 force field. The following implicit solvent (water) models considered here are: PCM (Polarized Continuum Model implemented in DISOLV and MCBHSOLV programs), GB (Generalized Born method implemented in DISOLV program, S-GB, and GBNSR6 stand-alone version), COSMO (COnductor-like Screening Model implemented in the DISOLV program and the MOPAC package) and the Poisson-Boltzmann model (implemented in the APBS program). Different parameterizations of the molecules were examined: we compared MMFF94 force field, Amber12 force field and the quantum-chemical semi-empirical PM7 method implemented in the MOPAC package. For small molecules, all of the implicit solvent models tested here yield high correlation coefficients (0.87–0.93) between the calculated solvation energies and the experimental values of hydration energies. For small molecules high correlation (0.82–0.97) with the explicit solvent energies is seen as well. On the other hand, estimated protein solvation energies and protein-ligand binding desolvation energies show substantial discrepancy (up to 10 kcal/mol) with the explicit solvent reference. The correlation of polar protein solvation energies and protein-ligand desolvation energies with the corresponding explicit solvent results is 0.65–0.99 and 0.76–0.96 respectively, though this difference in correlations is caused more by different parameterization and less by methods and indicates the need for further improvement of implicit solvent models parameterization. Within the same parameterization, various implicit methods give practically the same correlation with results obtained in explicit solvent model for ligands and proteins: e.g. correlation values of polar ligand solvation energies and the corresponding energies in the frame of explicit solvent were 0.953–0.966 for the APBS program, the GBNSR6 program and all models used in the DISOLV program. The DISOLV program proved to be on a par with the other used programs in the case of proteins and ligands solvation energy calculation. However, the solution of the Poisson-Boltzmann equation (APBS program) and Generalized Born method (implemented in the GBNSR6 program) proved to be the most accurate in calculating the desolvation energies of complexes.
 Ссылка на публикацию
Ссылка на публикацию
Katkova et al. Accuracy comparison of several common implicit solvent models and their implementations in the context of protein-ligand binding. Journal of Molecular Graphics and Modelling, 72:70–80, 2017.
Новости
А.А. Герасимова отмечена премией Правительства Москвы24 января 2026 17:55Заседание Ученого совета НИВЦ МГУ, 15 января 2026 г.30 декабря 2025 15:11А.В. Дебольскому присуждена степень кандидата физико-математических наук24 декабря 2025 16:26Торжественное заседание Ученого совета НИВЦ МГУ, 25 декабря 2025 г.21 декабря 2025 13:32Журнал НИВЦ
Контакты
119234, Российская Федерация, Москва, ГСП-1, Ленинские горы, дом 1, стр. 4, НИВЦ МГУ
+7 495 939-5424,
Подробнее
Новое на сайте
А.А. Герасимова отмечена премией Правительства Москвы24 января 2026Заседание Ученого совета НИВЦ МГУ, 15 января 2026 г.30 декабря 2025
ОБЛАКО ТЕГОВ
3D изображения 270-летие Московского университета 300 лет РАН Земная система Ломоносовские чтения Минобрнауки Научная Россия Научно-методологический семинар НИВЦ МГУ РНФ Российский научный фонд СМУ Семинар Фонд автоматическое реферирование анализ информационных ресурсов аспиранты и студенты благодарность влияние климата вычислительно-информационные технологии вычислительные методы геофизические процессы государственная награда грант защита диссертации интервью информационные системы информационные технологии климатическая система компьютерная лингвистика конгресс конкурс конкурс РНФ конкурс статей конкурсы президенские конференция лекция марафон математическое моделирование международная конференция методы машинного обучения моделирование Земной системы молекулярное моделирование молекулярный докинг молодежный конкурс молодые сотрудники молодые ученые награждение наука наука и образование научный журнал научный конкурс образование параллельные вычисления персонализированная медицина почетная грамота премия премия Ломоносова производство лекарств промышленность публикация стипендия супекомпютер суперкомпьютер суперкомпьютерное моделирование суперкомпьютерные дни суперкомпьютерные технологии суперкомпьютерный комплекс суперкомпьютерный центр МГУ ученый совет цифровизация школа школа-семинар экология экспериментальная лингвистика эстафета
Все материалы сайта НИВЦ МГУ доступны по лицензии:







